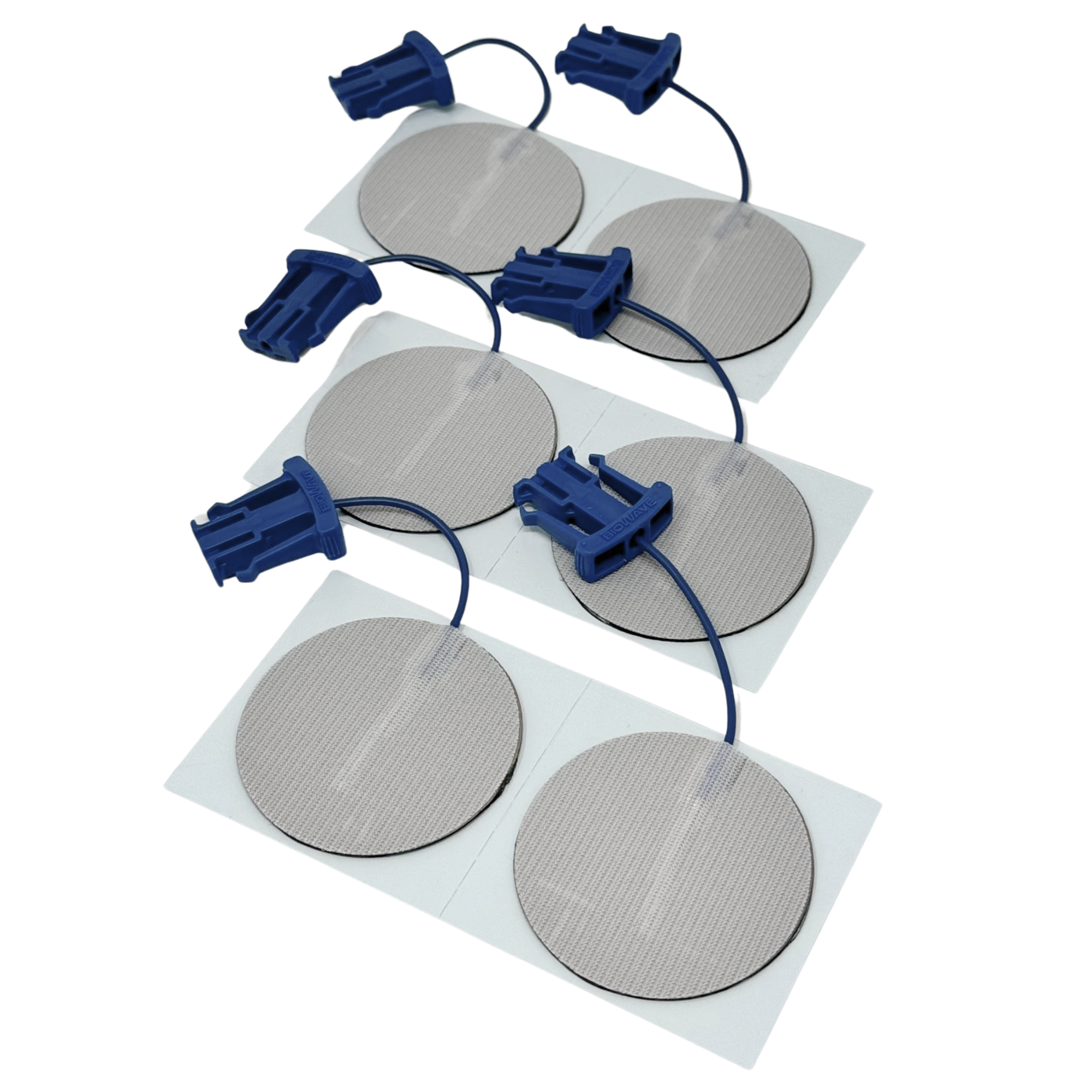
Signal-Mixing Technology
BioWaveGO, BioWaveHOME and BioWavePRO neurostimulators all utilize the same unique signal-mixing technology to deliver electrical signals through skin into deep tissue to the surface of nerves for inhibiting pain transmission and improving function. The signal technology is covered by numerous U.S. and international patents.
More Than 84% Of Patients Respond To BioWave
Multiple clinical studies show that more than 84% of patients experience a significant reduction in pain.* It literally gets them “back in the game”. BioWave neurostimulators can be used to reduce chronic, acute or post-operative pain and improve function including an increase in range of motion, decrease in stiffness and reduction of muscle spasm for an extended period of time following a 30 minute treatment.
Pick A Body Part – Any Body Part
BioWave neurostimulators can be used to treat numerous locations on the body including directly over or surrounding the sacral, lumbar, thoracic and cervical spine, hip, groin, knee, shoulder, ankle, foot, elbow, wrist, hand, and finger. Doctors, physical therapists, and athletic trainers report excellent results treating pain from acute and chronic tendinopathies, joint sprains, trigger points, osteoarthritis, pelvic floor pain including interstitial cystitis and post-operative pain.
BioWave Scores Big With Athletes
Professional athletes that use BioWavePRO, BioWaveHOME, or BioWaveGO have reported they experience better pain relief and functional improvement than with any other form of electrical stimulation and request to be treated with BioWave every time they return to the training room, while in meetings, or at home.
Clinical Studies And Reviews
BioWave is as well-researched and well-documented as it is well-received. Read the studies that have helped make BioWave the patented, proven, smarter pain blocking technology.
Reduced Pain and Improved Function Following Short-Term Use of Noninvasive BioWave High Frequency Peripheral Nerve Stimulation for Pain Management
Alaa Abd-Elsayed, Michael Gyorfi, Michael Fischman, Charles Odonkor, Bradford Siff, Kevin Cyr. Pain and Therapy; Feb 20, 2023.
Open-label survey of chronic pain patients recruited from Veteran Affairs, orthopedic and pain health systems. This retrospective pilot study is shaped around a noninvasive neuromodulation system over a 2-week treatment timeline. The magnitude of the patient population (1511 patients) is amongst the largest population sizes ever studied for noninvasive neuromodulation. The study aimed at assessing the efficacy of non-invasive peripheral nerve stimulation using BioWave to treat chronic pain.
An Open-Label Pilot Study InvestigatingNoninvasive High-Frequency Peripheral NerveFiber Stimulation in Chronic Pain
D.A. Hegarty, B. Bretherton. Pain Management and Neuromodulation, Mater Private Hospital, Cork;
Department of
Anesthesiology, School of Medicine, University College, Cork, Ireland;
School of Biomedical
Sciences, Faculty of Biological Sciences, University of Leeds, Leeds;
Pain Management
Department, Leeds Teaching Hospitals NHS Trust, Leeds, UK
Post-operative pain treatment following total knee replacement surgery using BioWavePENS
T. Wanich, J. Gelber, R. Windsor, S. Rodeo. A randomized controlled pilot study to determine average maximum intensity settings, safety, and initial efficacy in terms of pain reduction, increased range of motion, and reduced pain medications, for a percutaneous neuromodulation pain therapy device (“BioWavePENS®”) following post-operative treatments for total knee replacement procedures.
Treatment of chronic pain from osteoarthritis of the knee using BioWavePENS
Brian J. Cole, MD, MBA; Richard Kang, MD, MS; Paul B. Lewis, MD, MS; Adam Kramer, ATC; Jennifer K. Hayden, RN, MSN; Randomized Single-blinded Controlled Clinical Trial of a BiowavePENS device (Percutaneous Electrical Nerve Stimulation) versus Sham PENS treatment for the Osteoarthritic Knee. Orthopedics, June 2007, Volume 30, Number 6.
Treatment of pain from acute sports injuries using BioWavePRO
S. Panchal, J. Pergolizzi. White Paper on use of a neuromodulation pain therapy device (“BioWavePRO”) to treat acute sports injuries on New York Giants football players. 600 treatments on 80 players from June 2003 – December 2005. Unpublished manuscript, 2006.
Treatment of joint pain using BioWaveHOME
W. Coren. A clinical study of the efficacy of the BioWave home device for reducing pain and increasing range of motion over 24 hours following a 20-minute treatment of shoulder, knee, hip, and extremity pain. Unpublished manuscript, 2003.
Evaluation of optimal signal frequency and efficacy of BioWave
S. Diwan, H.C. Hemmings, R.F. Eliazo, S. Panchal. Symptomatic treatment of chronic low back pain: determination of optimal signal frequency and preliminary efficacy of a targeted noninvasive electronic pain control device. Anesth. Analg. Abstracts 96 S-1-S: 293, 2003.
Randomized, Double-Blinded, Comparative Crossover Trial Comparing Biowave vs TENS
S. Diwan MD, H. Hemmings MD, PhD, S. Panchal MD, Summary of a Randomized, Double-Blinded, Comparative Crossover Trial of the Safety and Efficacy of a Non-Invasive Targeted Electronic Pain Control Device (“Biowave System”) Versus Transcutaneous Electrical Nerve Stimulation (TENS) for the Symptomatic Treatment of Chronic Low Back Pain; March 2006.
Long Term Use of BioWaveHOME for the Treatment of Chronic Pain in Veterans
L. Kleinwaks, M. Rogers, D. McGurl.Long Term Use of BiowaveHOME High Frequency Neurostimulation for the Treatment of Chronic Pain in Veterans Following Successful BiowavePRO Neurostimulation Therapy in Veterans Administration Hospitals. White Paper, October 2017.
Neuromodulation Pain Therapy Treatment
R. Yacoub. Neuromodulation pain therapy. ADVANCE for Physical Therapy and Rehab Medicine 23(13): 22-24, 2012.
Significantly Reduced Pain and Improved Function Following Short-Term Use of the BioWaveHOME High Frequency Neurostimulation System for Conservative Pain Management
Lindsay Kleinwaks, Ph.D., Senior Associate, Regulatory Affairs Dave McGurl, Director, Regulatory Affairs. Following two weeks of BioWaveHOME use, four hundred sixty three (463) subjects provided responses via returned surveys. The BioWaveHOME device provided subjects with statistically and clinically significant reductions in pain, with an average reduction in pain score of 3 points.